More Information
Submitted: October 30, 2024 | Approved: November 22, 2024 | Published: November 25, 2024
How to cite this article: Gökhan C, Ümit A, Lezgin D, Muhammet A, Cüneyt S. Closure of Post-infarct Basal Ventricular Septal Defect by Using an Atrial Septal Defect Closure Device: A Case Report. J Clin Med Exp Images. 2024; 8(1): 013-016. Available from: https://dx.doi.org/10.29328/journal.jcmei.1001033.
DOI: 10.29328/journal.jcmei.1001033
Copyright License: © 2024 Gökhan C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: VSD; Closure device; Post-mi complications; Post-infarct
Closure of Post-infarct Basal Ventricular Septal Defect by Using an Atrial Septal Defect Closure Device: A Case Report
Ceyhun Gökhan1*, Arslan Ümit2, Dursun Lezgin3, Aydin Muhammet4 and Şeker Cüneyt5
1Department of Cardiology, Faculty of Medicine and Research Hospital, Ataturk University, Turkey
2Department of Cardiovascular Surgery, Faculty of Medicine and Research Hospital, Ataturk University, Turkey
3Department of Cardiology, Bingöl Public Hospital, Turkish Republic Ministry of Health, Turkey
4Department of Anesthesiology and Reanimation, Ataturk University, Turkey
5Department of Cardiology, Ataturk University, Turkey
*Address for Correspondence: Ceyhun Gökhan, Department of Cardiology, Faculty of Medicine and Research Hospital, Ataturk University, Erzurum, Turkey, Email: [email protected]
Ventricular Septal Defect, also known as VSD is a rare and life-threatening complication associated with MI. Therefore, it should be immediately diagnosed and treated. Transcatheter closure of the ventricular septal defect is a new alternative treatment approach compared to surgery. In this case, we presented a patient with post-infarct basal ventricular septal defect whose ventricular septal defect was closed using an atrial septal defect closure device. The ability to successfully close such a large defect via catheter is promising for the treatment of patients with VSD.
Rupture of the interventricular septum is a rare and life-threatening complication of acute myocardial infarction, especially in patients with ST-Segment Elevation Myocardial Infarction (STEMI). Like STEMI, non-STEMI can rarely lead to this fatal complication. In a retrospective analysis, the incidence of Ventricular Septal Defect (VSD) associated with STEMI has been determined %0.21. According to this study, VSD-AMI was the most common mechanical complication of STEMI and this condition has been diagnosed among patients with ST-segment elevation myocardial infarction more than among patients with non-STEMI (%0.21 and %0.04, respectively) [1]. This complication has high in-hospital mortality rates. In a retrospective analysis, 30-day mortality rates of VSD-AMI and 1-year mortality have been determined as %60.2 and %68.5, respectively [2]. We have two options for the treatment of VSD: surgically or percutaneously. In this case, we presented a patient with VSD associated with inferior myocardial infarction whose basal VSD had been closed successfully using an Atrial Septal Defect (ASD) closure device.
Differential diagnosis
If a patient with myocardial infarction, especially STEMI, has an impaired hemodynamic status or incompatible symptoms like dyspnea and decline in consciousness although complete recanalization of the culprit lesion, it should be suspected of a complication, especially mechanical complications. These complications include partial and complete papillary muscle rupture, ventricular septal defect, and freewall rupture. These conditions require an immediate physical examination and an echocardiography. Because mechanical complications associated with STEMI are life-threatening conditions that can lead to death immediately. To be heard a holosystolic murmur at the left parasternal site on physical examination and to be demonstrated a shunt from LV to RV on echocardiography are diagnostic for ventricular septal rupture.
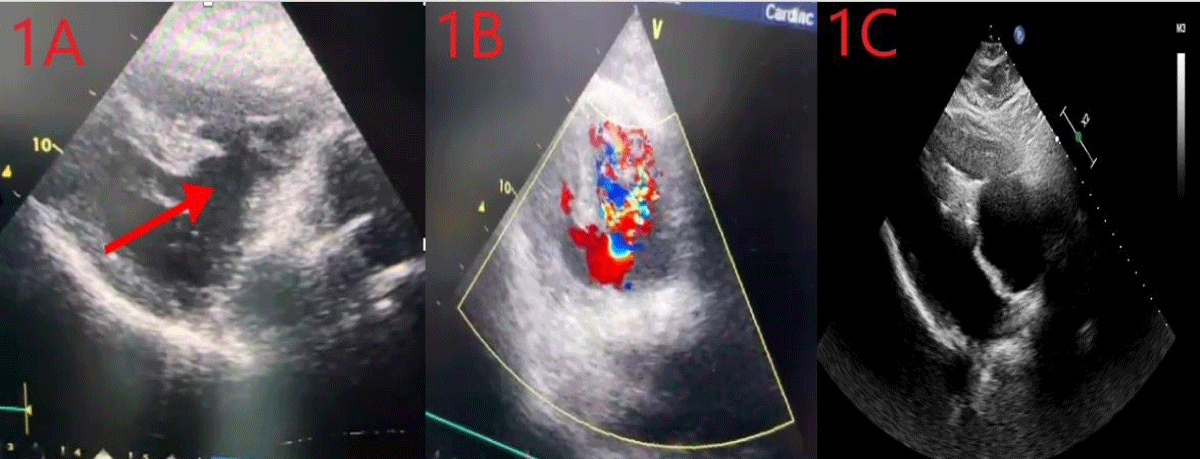
A 65-year-old male patient who has priorly chronic obstructive lung disease and hypertension was referred to our heart center from another hospital due to VSD – post STEMI. The patient was feeling dyspnea and the patient’s NYHA classification was class 3. The patient’s hemodynamic status was stable. There was a holosystolic murmur in the left fourth intercostal space on physical examination. We urgently performed transthoracic echocardiography with 2D imaging. Echocardiographic imaging revealed an aneurysmatic VSD at the basal level of the interventricular septum, and color doppler imaging revealed passage through the interventricular septum. (Image 1A,B). We made a joint evaluation with cardiovascular surgeons for the patient’s surgical treatment. Because of the high perioperative mortality rates due to anatomical localization, we decided to perform percutaneous closure in mutual agreement with the Department of Cardiovascular Surgery. This case was conducted in accordance with the 1975 Declaration of Helsinki and its 1983 revision. Informed consent was obtained from the patient.
Image 1: A: Transthoracic echocardiographic image at the time of admission, B: The transition between both ventricles is visible, C: Image after the release of ASD device.
Technique
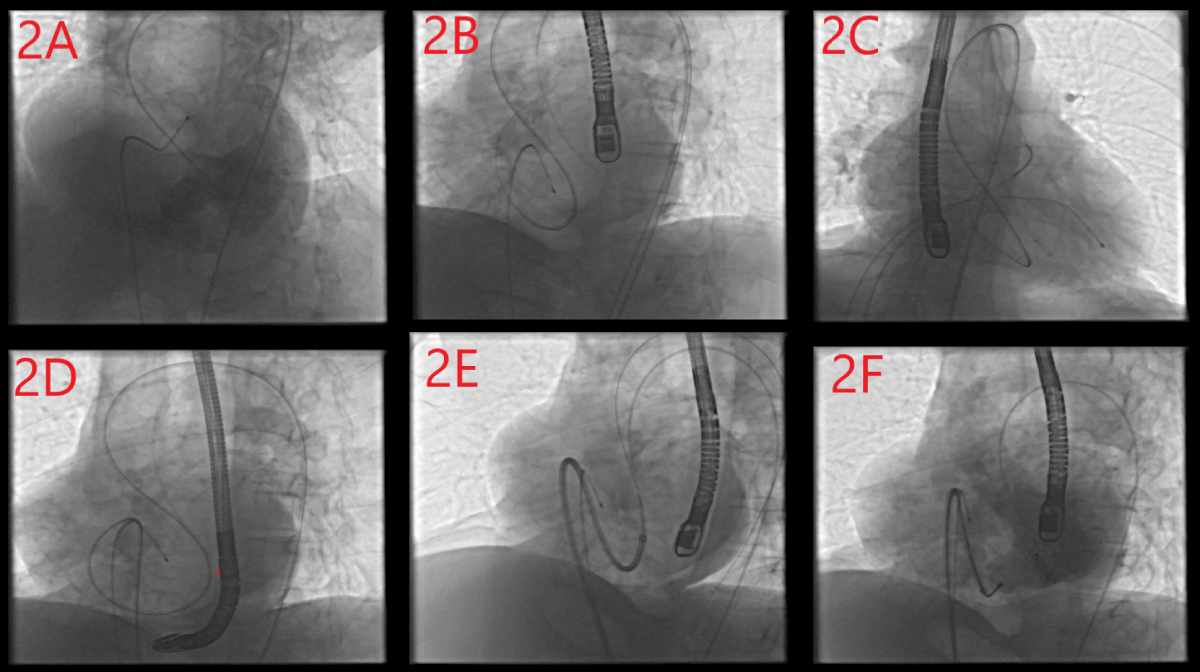
The patient was admitted to the procedure under light sedation. The procedure was applied by an anesthesiologist. During the procedure, 2D transesophageal echocardiography was used to enhance procedural imaging support. Bilateral femoral vein and arterial accesses were achieved to facilitate the transition of adequate equipment. The anatomical location of the VSD was determined angiographically by placing a pigtail catheter into the left ventricle via the left femoral artery. Left ventriculography was performed to measure the defect diameter (Image 2A). The defect diameter was 23.5 mm. A temporary transvenous pacemaker was placed in the right ventricle to suppress catheter-induced arrhythmias due to the risk of AV block that may occur after the device was placed. Ventricular capture was made with high output during the process. The 7F right coronary guide catheter was advanced to the left ventricle over the 0.035-inch hydrophilic guidewire, and the guidewire and catheter were advanced from the left ventricle to the right ventricle via the VSD. Then, the guide wire was advanced to the right ventricular outflow tract (Image 2B). A 6F right coronary guiding catheter was advanced to the right ventricular outflow tract via right femoral vein access. Then, the hydrophilic guidewire was snared into the 6F right guiding catheter in the pulmonary artery using a vascular snare (Image 2C). Thus, the arteriovenous loop was achieved. The hydrophilic guidewire which was advanced from right femoral arterial access was removed from the right femoral vein access. We advanced the right guide catheter coming from the femoral vein over the hydrophilic guide wire, past the VSD and towards the aorta, and removed the hydrophilic guide wire (Image 2D). We advanced an amplatz super stiff 0.035-inch guidewire to the aorta through the 6F right guiding catheter via right femoral vein access and removed the catheter. The 10-French wide access catheter, which enabled the closure device to be advanced, was advanced to the LVOT via the VSD over the stiff guidewire (Image 2E). Then, the amplatz super-stiff guidewire was removed. 28 mm ASD closure device priorly prepared was advanced to the tip of the 10F sheath placed LVOT. The left disc of the device was opened on the left ventricle side, and the right disc of the device was opened on the right ventricle side (Image 2F). Left ventriculography and transesophageal echocardiography were performed to determine whether there was a residual shunt or not. There wasn’t a shunt on ventriculography and TEE (Image 1C). We released the device and the process was completed successfully. No complications developed during the procedure. After the procedure, the patient’s hemodynamic status was stable and there was no symptom associated with VSD. Following the five days in the coronary intensive care unit and fifteen days of inpatient clinic monitoring, liver and kidney function indicator laboratory parameters returned to normal.
Image 2: A: Angiographic image of the defect, B: Transition from left ventricle to right ventricular outflow tract with hydrophilic wire, C: Taking the hydrophilic wire in the right ventricular outflow tract into the catheter coming from the femoral vein with a snare, D: Creation of arteriovenous loop, E: Sending the device access catheter to the left ventricular outflow tract via the stiff wire, F: Positioning the device.
Mechanical complications of myocardial infarction are rare but life-threatening. These conditions should be diagnosed and treated immediately. One of these conditions is ventricular septal defect and it is one of the most seen mechanical complications of MI. The most experienced and traditionally applied method in the treatment of VSD is surgical repair. Surgical repair has high perioperative mortality rates. This rate varies depending on the patient’s hemodynamics, the width of the defect, and its anatomical location, but it is between 20% - 87% [3,4]. Recently, device closure methods have been used as an alternative to surgical repair. Percutaneous intervention should be kept in mind, especially in patients with variable anatomical localizations and hemodynamic disorders that cause them to not be able to tolerate surgery. However, research and case series on device closure methods are limited [5-10]. It is understood from studies conducted with a few patients that defects, especially those that are large and located in the basal septum, are more mortal. This may be because the ventricular outflow tract device affects placement and the atrioventricular node is under stress. During the procedure, we encountered a complete A-V block and ventricular arrhythmias. To overcome this, we used high-output pacing. It is noteworthy that our patient didn’t have hemodynamic compromise despite the large defect diameter and shunt. We thought that this situation was related to right ventricular hypertrophy associated with COPD. Thus, the volume load on the right ventricle could be tolerated. However, there is not enough data about the technique to be used for closure, procedural timing, preoperative and postoperative follow-up, and medical treatment.
Author contributions
Ceyhun G. Case management; Arslan Ü and Şeker C. manuscript preparation; Dursun L. and Aydın M. Echocardio-graphy support.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
- Elbadawi A, Elgendy IY, Mahmoud K, Barakat AF, Mentias A, Mohamed AH, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12:1825-1836. Available from: https://doi.org/10.1016/j.jcin.2019.04.039
- Goldsweig AM, Wang Y, Forrest JK, Cleman MW, Minges KE, Mangi AA, et al. Ventricular septal rupture complicating acute myocardial infarction: incidence, treatment, and outcomes among Medicare beneficiaries 1999–2014. Catheter Cardiovasc Interv. 2018;92:1104-1115. Available from: https://doi.org/10.1002/ccd.27576
- Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, et al. Post infarction ventricular septal defect: can we do better? Eur J Cardiothorac Surg. 2000;18:194-201. Available from: https://doi.org/10.1016/s1010-7940(00)00482-6
- Jeppsson A, Liden H, Johnsson P, Hartford M, Radegran K. Surgical repair of post-infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg. 2005;27:216-221. Available from: https://doi.org/10.1016/j.ejcts.2004.10.037
- Ahmed J, Ruygrok PN, Wilson NJ, Webster MWI, Greaves S, Gerber I. Percutaneous closure of post-myocardial infarction ventricular septal defects: a single center experience. Heart Lung Circ. 2008;17:119-223. Available from: https://doi.org/10.1016/j.hlc.2007.09.001
- Ialkowski J, Szkutnik M, Kusa J, Kalarus Z, Gasior M, Przybylski R, et al. Transcatheter closure of postinfarction ventricular septal defects using amplatzer devices. Rev Esp Cardiol Engl Ed. 2007;60:548-551. Available from: https://pubmed.ncbi.nlm.nih.gov/17535768/
- Martinez MW, Mookadam F, Sun Y, Hagler DJ. Transcatheter closure of ischemic and post-traumatic ventricular septal ruptures. Catheter Cardiovasc Interv. 2007;69:403-407. Available from: https://doi.org/10.1002/ccd.20949
- Chessa M, Carminati M, Cao Q-L, Butera G, Giusti S, Bini RM, et al. Transcatheter closure of congenital and acquired muscular ventricular septal defects using the Amplatzer device. J Invasive Cardiol. 2002;14:322-327. Available from: https://pubmed.ncbi.nlm.nih.gov/12042624/
- Thiele H, Kaulfersch C, Daehnert I, Schoenauer M, Eitel I, Borger M, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30:81-88. Available from: https://doi.org/10.1093/eurheartj/ehn524
- Lee KT, Taliotis D, Hamilton M, Manghat N, Bedair R, Turner M. Transcatheter closure of large postinfarct ventricular septal defect: Initial results of prototype Occlutech® device including the first-in-human. Catheter Cardiovasc Interv. 2023;101:620–27. Available from: https://doi.org/10.1002/ccd.30571